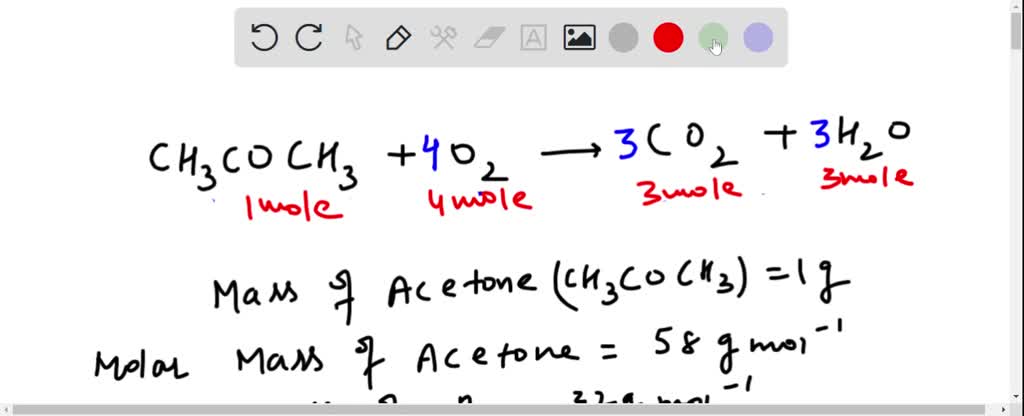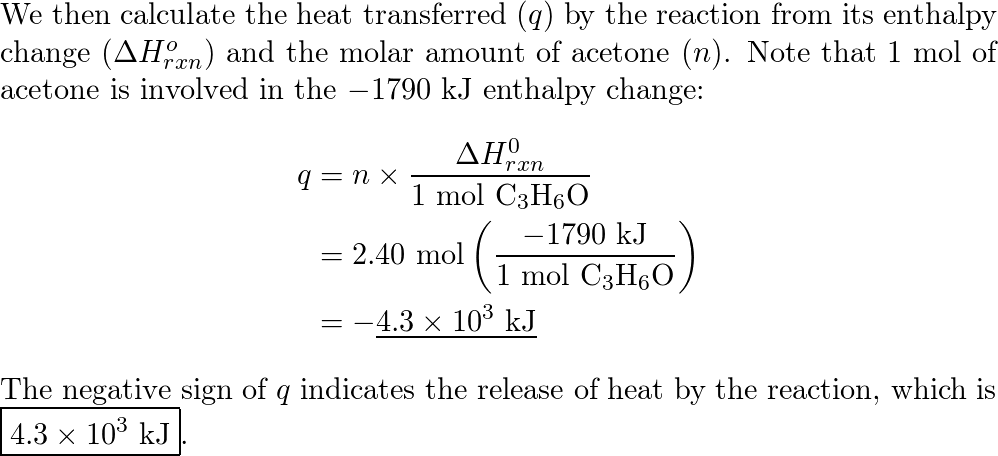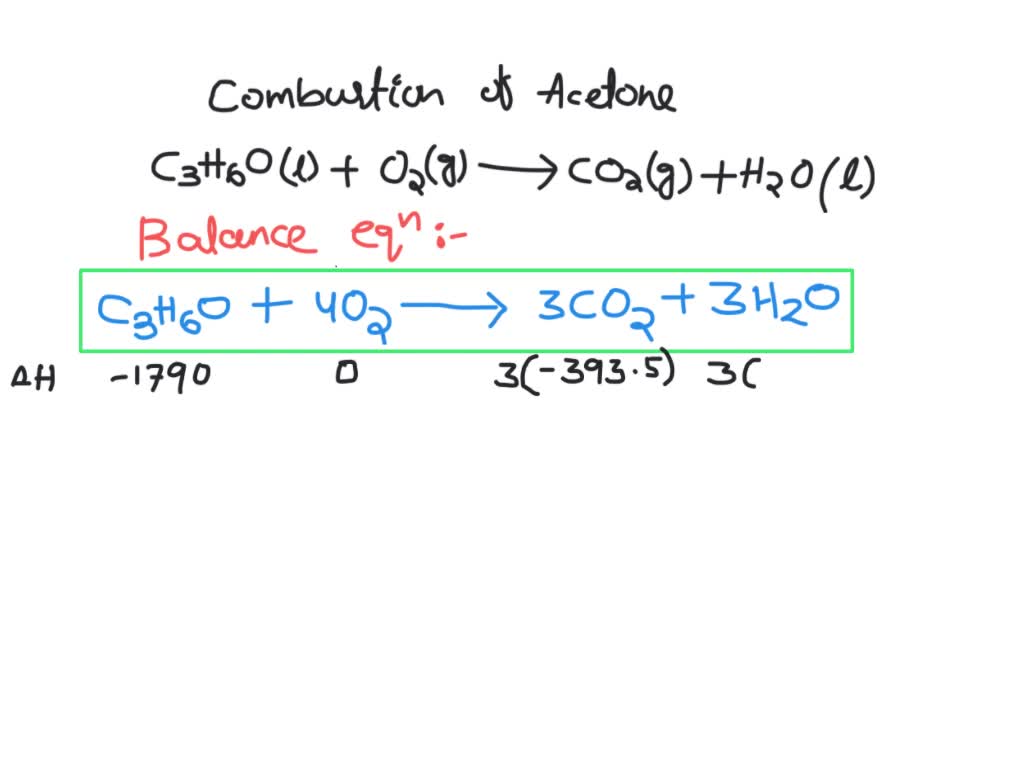
SOLVED: 5.4 Complete combustion of 1.00 mol of acetone (C3H6O) liberates 1790 kJ: C3H6O(l) + O2(g) â†' CO2(g) + H2O(l) (unbalanced) 5.4.1 Write the balanced thermochemical equation for the reaction. 5.4.2 Use

Calculate the volume of CO2 produced by the combustion of 40 mL of acetone in the presence of excess of oxygen.
Catalysts | Free Full-Text | Valorization of Recycled Honeycombs from Exhausted TWCs by Means of Their Use as a Support of MnOx Catalysts for Acetone Combustion
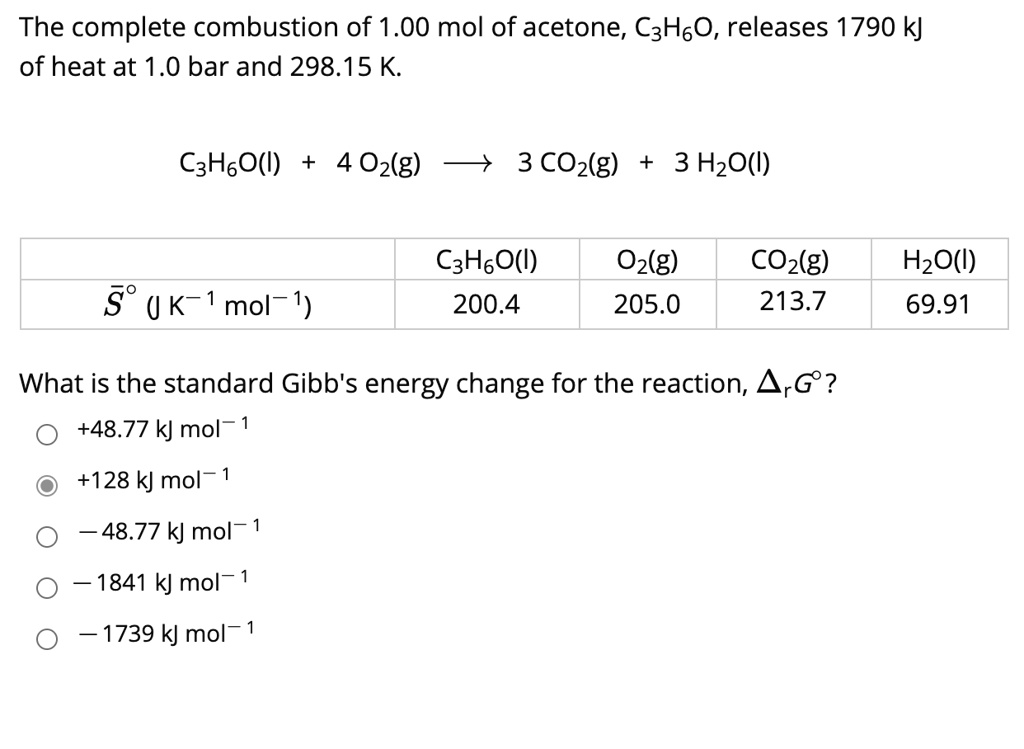
SOLVED: The complete combustion of 1.00 mol of acetone, C3H6O, releases 1790 kJ of heat at 1.0 bar and 298.15 K. C3H6O(g) + 4 O2(g) -> 3 CO2(g) + 3 H2O(g) C3H6O(g)

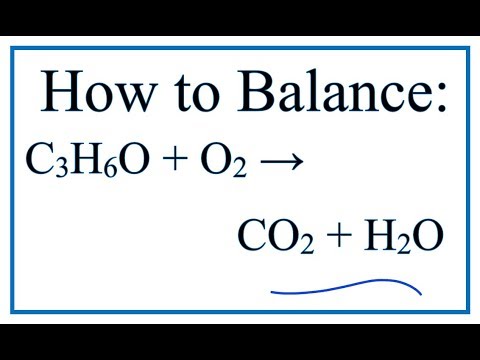
A) balance the equation B) if a bottle of nail polish remover contains 155g of acetone how much heat is - brainly.com
Kinetic analysis of the acetone-butanol-ethanol combustion mechanism in 0D simulated Otto cycle internal engine | SpringerLink

Instantaneous acetone-PLIF images for the combustion case recorded at a... | Download Scientific Diagram

The volume of `CO_2` prodcued by the combination of 40 ml of gaseous acetone in excess of oxygen is - YouTube

SOLVED: Consider the following reaction involving the combustion of acetone (C3H6O): C3H6O (L) + 4 O2 (g) â†' 3 CO2 (g) + 3 H2O (l) ΔH = 1790 kJ. How much heat (