Frontiers | IgG Anti-Spike Antibodies and Surrogate Neutralizing Antibody Levels Decline Faster 3 to 10 Months After BNT162b2 Vaccination Than After SARS-CoV-2 Infection in Healthcare Workers
Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays
Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays
Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays

WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: call for urgent action by the scientific community - The Lancet Microbe

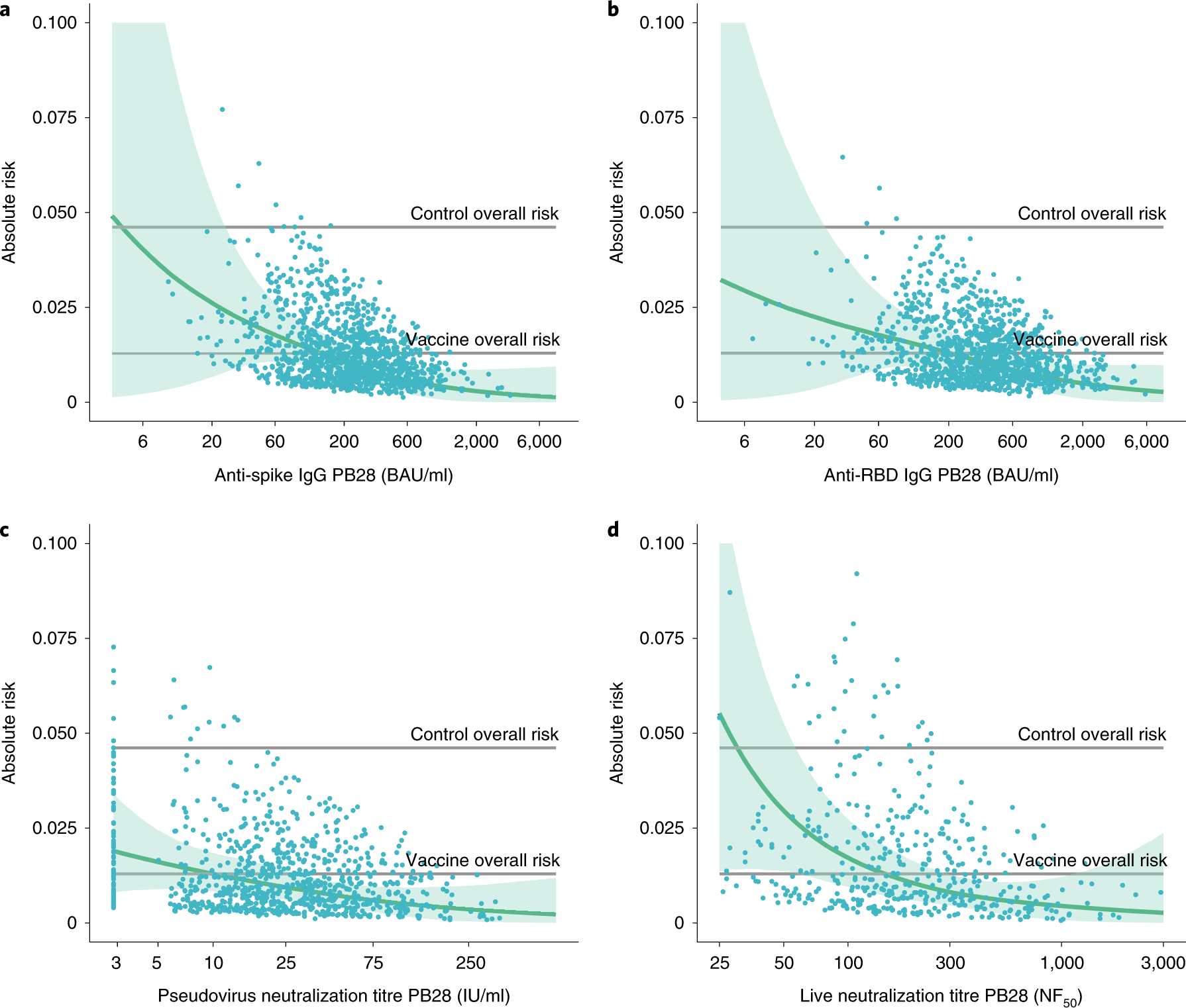
Calibrated comparison of SARS-CoV-2 neutralizing antibody levels in response to protein-, mRNA-, and vector-based COVID-19 vaccines | npj Vaccines
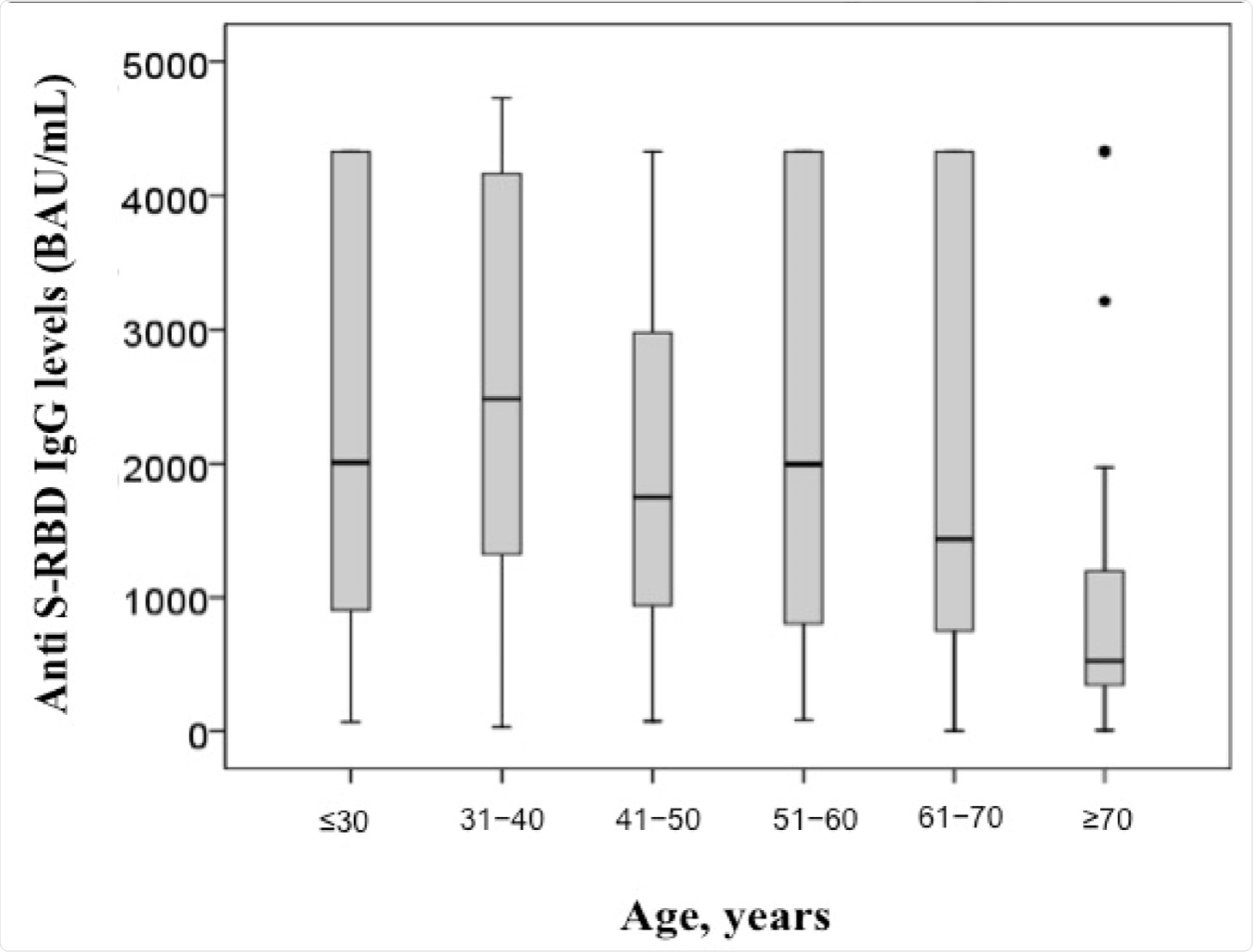
Vaccines | Free Full-Text | Robust SARS-CoV-2 Antibody Responses in Asian COVID-Naïve Subjects 180 Days after Two Doses of BNT162b2 mRNA COVID-19 Vaccine

The Comparability of Anti-Spike SARS-CoV-2 Antibody Tests is Time-Dependent: a Prospective Observational Study | Microbiology Spectrum
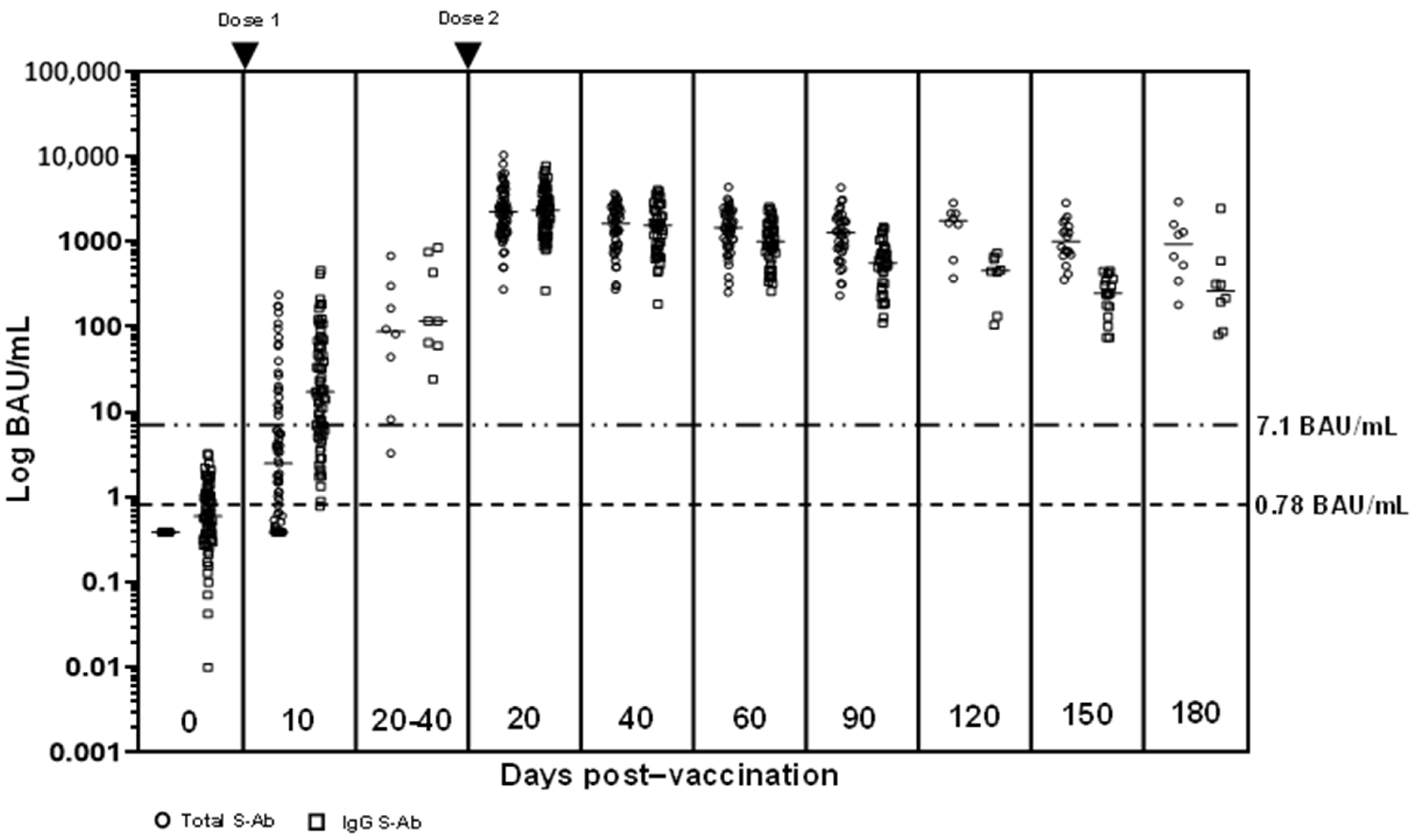
The comparability of Anti-Spike SARS-CoV-2 antibody tests is time-dependent: a prospective observational study | medRxiv

Anti-Spike protein assays to determine post-vaccination antibody levels: a head-to-head comparison of five quantitative assays | medRxiv

Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection - eBioMedicine
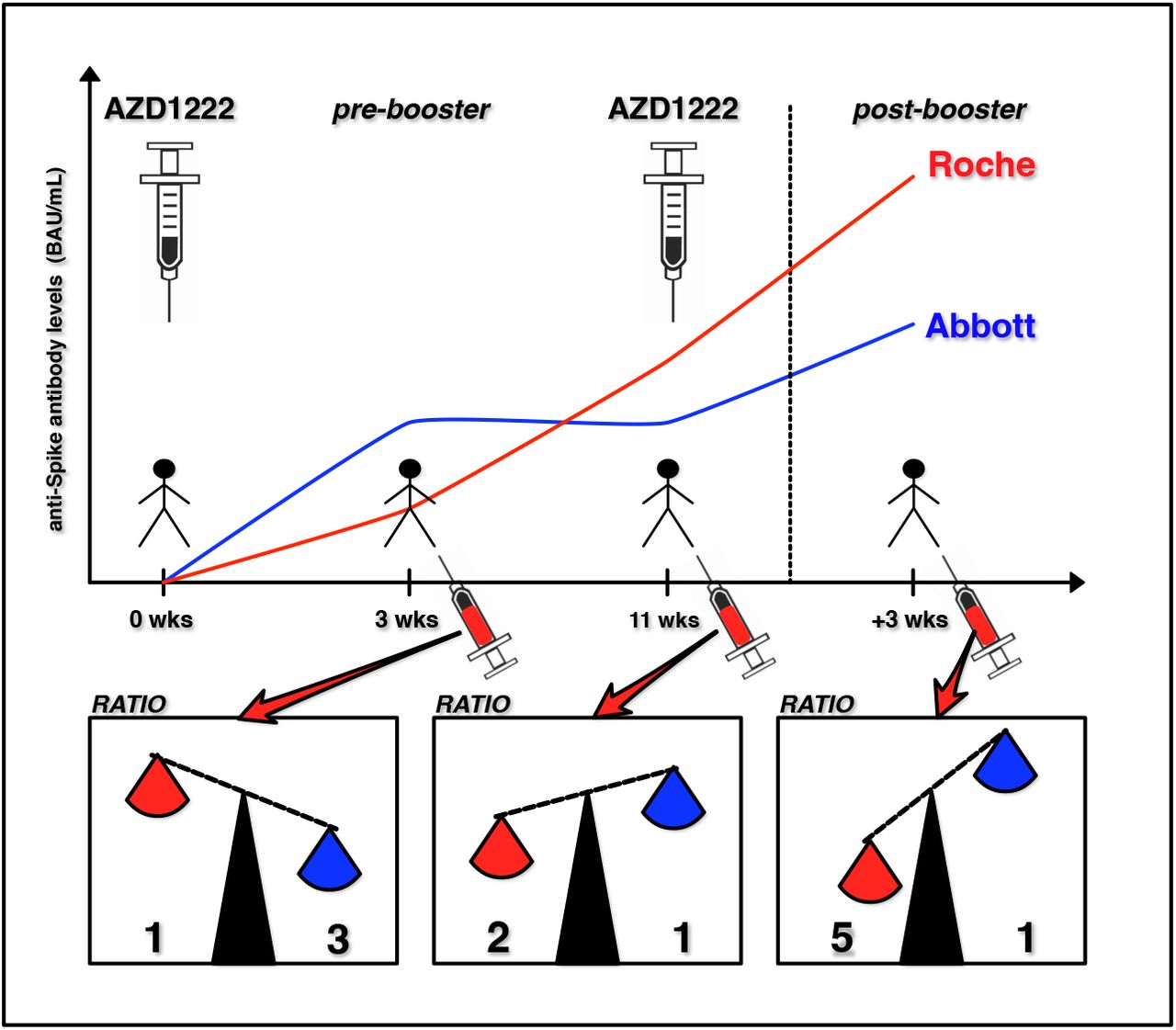
COMPARISON OF FIVE COMMERCIAL ANTI-SARS-COV-2 TOTAL ANTIBODIES AND IgG IMMUNOASSAYS AFTER VACCINATION WITH BNT162B2 MRNA

The Comparability of Anti-Spike SARS-CoV-2 Antibody Tests is Time-Dependent: a Prospective Observational Study | Microbiology Spectrum